Short Communication
Insights for Antihypertensive pharmacotherapy from the “Calcium Paradox” due to Ca2+/camp Interaction

Leandro Bueno Bergantin* and Afonso Caricati-Neto
Department of Pharmacology-Universidade Federal de São Paulo-Escola Paulista de Medicina, Brazil
*Address for Correspondence: Dr. Leandro Bueno Bergantin, Department of Pharmacology-Universidade Federal de São Paulo-Escola Paulista de Medicina, Laboratory of Autonomic and Cardiovascular Pharmacology-55 11 5576-4973, Rua Pedro de Toledo, 669-Vila Clementino, São Paulo-SP, Brazil, CEP: 04039-032; E-mail: [email protected]
Dates: Submitted: 06 March 2017; Approved: 24 March 2017; Published: 27 March 2017
How to cite this article: Bergantin LB, Caricati-Neto A. Insights for Antihypertensive pharmacotherapy from the “Calcium Paradox” due to Ca2+/camp Interaction. Ann Clin Hypertens. 2017; 1: 006-009. DOI: 10.29328/journal.ach.1001002
Copyright License: © 2017 Bergantin LB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ca2+/cAMP interaction; Sympathetic neurotransmission; Hypertension
ABSTRACT
Several experimental studies performed since 1975, using smooth muscles richly innervated by sympathetic nerves to exclude the autonomic influence of adjusting reflex (rodent vas deferens), showed that L-type voltage-activated Ca2+ channels (VACC) blockers completely inhibited neurogenic contractions induced by electrical field stimulation (EFS) in high concentrations (>10-6 M), but paradoxically increased these EFS-contractions in low concentrations (<10-6 M), suggesting that other mechanisms than only autonomic adjusting reflex are involved in these paradoxical effects. In 2013, we showed that these paradoxical effects of L-type VACC blockers, named by us “calcium paradox” phenomenon, were potentiated by drugs which increase cytosolic cAMP concentration ([cAMP] c-enhancers), such as rolipram, IBMX and forskolin, indicating that this sympathetic hyperactivity drug-induced is due to interaction of the Ca2+/cAMP intracellular signaling pathways (Ca2+/cAMP interaction). Then, the pharmacological manipulation of this interaction produced by combination of the L-type VACC blockers used in the antihypertensive therapy, and [cAMP] c-enhancers used in the antidepressive therapy, could represent a potential cardiovascular risk for hypertensive patients due to sympathetic hyperactivity. Then, we discussed the role of Ca2+/cAMP interaction for antihypertensive pharmacotherapy.
INTRODUCTION
Since 1970´s, several clinical studies have reported that acute and chronic administration of Ca2+ channels blockers (CCBs) in hypertensive patients, such as verapamil and nifedipine, produces reduction in peripheral vascular resistance, and arterial pressure, due to vasodilation resultant of reduction of Ca2+ influx through plasmalemal L-type voltage-activated Ca2+ channels (VACC) in smooth muscle cells, but it also produces increase in plasma catecholamines levels and heart rate, typical symptoms of sympathetic hyperactivity [1]. Despite the risks of anti-hypertensive therapy, these adverse effects of L-type VACC blockers were initially attributed to autonomic adjust reflex of arterial pressure. However, several experimental studies performed in 1970´s, by studying the effects of L-type VACC blockers in smooth muscle richly innervated by sympathetic neurons (rodent vas deferens) submitted to electrical field stimulation (EFS), demonstrated that these blockers could produce dual effect on sympathetic neurotransmission. In high concentrations (>1 µM), L-type VACC blockers completely inhibited sympathetic EFS-contractions due to blockade of Ca2+ influx in smooth muscle cells, but paradoxically increased these contractions in low concentrations (<1 µM), suggesting that other mechanisms than only autonomic adjusting reflex are involved in these paradoxical effects. However, the cellular and molecular mechanisms involved in these paradoxical effects of L-type VACC blockers remained unclear during almost four decades [2-5].
Theoretical model of Ca2+/cAMP interaction in neurons and neuroendocrine cells
In a study published in 2013 in Cell Calcium, we showed that this paradoxical increase of sympathetic activity produced by L-type VACC blocker, named by us as “calcium paradox”, is resultant of interference produced by these blockers on the interaction of intracellular signaling pathways mediated by Ca2+ and cAMP (Ca2+/cAMP interaction) [6]. We showed that simultaneous administration of L-type VACC blockers (verapamil) with drugs which produce increase of cytosolic cAMP concentration ([cAMP] c-enhancers), such as rolipram, IBMX and forskolin, increased purinergic component of sympathetic EFS-contractions of rat vas deferens [6]. In addition, this increase was prevented by reduction of [cAMP]c caused by adenylyl cyclase (AC) inhibition by SQ 22536, or depletion of Ca2+ storage of endoplasmic reticulum (ER) by thapsigargin, revealing the involvement of Ca2+ mobilization from ER in this response [6].
Our studies showed that paradoxical increase of sympathetic activity produced by Ca2+ channels blockers, used in anti-hypertensive therapy, results from increment of neurotransmitters release from secretory response of postganglionic sympathetic neurons due to its interference on the Ca2+/cAMP interaction [6-8]. Thus, reduction of Ca2+ influx in these cells by verapamil and other Ca2+ channels blockers (in low concentration) increases AC activity, and [cAMP]c, due to decrease of [Ca2+]c [6-8]. It was showed that 5 and 6 isoforms of AC are involved in this AC-inhibition by Ca2+. The [cAMP]c increment leads to increment of cAMP-stimulated Ca2+-release from ER, that in turn, enhances exocytotic process due to increase of secretory vesicles recruitment, and docking, to the plasma membrane, priming of fusion machinery, and fusion of vesicles with the plasma membrane [6-8].
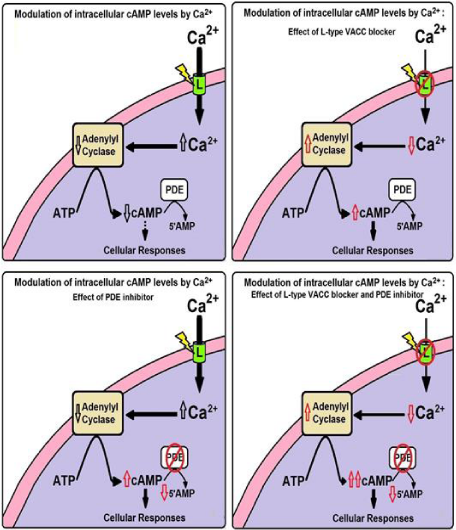
Theoretical model of Ca2+/cAMP interaction in secretory cells proposed by Bergantin et al. (2013) and Caricati-Neto et al. (2015) illustrates how secretory responses of the neurons and neuroendocrine cells can be modified by pharmacological modulation of intracellular levels of Ca2+ and/or cAMP (Figure 1). This proposement is reforcing the hypothesis that Ca2+/cAMP interaction plays an important role in the sympathetic activity, by regulating secretory response of sympathetic neurons, and adrenal chromafin cells [6]. In addition, these pharmacological manipulations of the Ca2+/cAMP interaction could have important implications for antihypertensive pharmacotherapy [7,8].
Figure 1: Theoretical model of Ca2+/cAMP interaction in neurons and neuroendocrine cells proposed by Bergantin et al. [6] and Caricati-Neto et al. [8]. This model illustrates how cellular responses of cells can be modified by pharmacological modulation of intracellular levels of Ca2+ and/or cAMP.
Implications of Ca2+/cAMP interaction for antihypertensive pharmacotherapy
Considering Medline database from 1975 to 1996, in which Grossman and Messerli (1998) [1] found 63 clinical studies involving 1,252 hypertensive patients reporting sympathetic hyperactivity produced by acute and chronic administration of L-type VACC blockers, and also other reports in some hypertensive patients that nifedipine has been reported to cause sympathetic activation and a paradoxical augmentation of blood pressure [9,10]. Then, whether this “calcium paradox” due to Ca2+/cAMP interaction is involved in this sympathetic hyperactivity in hypertensive patients deserves special attention.
In fact, L-type VACC blockers like verapamil, and nifedipine analogous, have been extensively used to reduce blood pressure in hypertensive patients, especially in combination with other drugs for treating angina or cardiac arrhythmias [11]. In the field of drug interaction, we could also infer that a therapy involving the combination of VACC blockers with drugs which increase [cAMP]c should be done carefully in hypertensive patients with neurological/psychiatric disorders [12], considering the role of sympathetic transmission in regulating vascular tone by releasing neurotransmitters into the vasculature. Then, this pharmacological interference of the Ca2+/cAMP interaction could represent a potential risk for antihypertensive therapy due to increase in sympathetic hyperactivity in the cardiovascular system. As future perspectives, it would be interesting to analyze these effects in mice with a knockout of PDE variants and/or other steps of the signalling pathways. These complemmentary results could strength the theoretical model of Ca2+/cAMP interaction.
CONCLUSION
In conclusion, Ca2+/cAMP interaction participates in the regulation of secretory response of sympathetic neurons and adrenal chromafin cells, and alterations of this interaction could be involved in sympathetic hyperactivity of hypertension due to exacerbation of catecholamines, and purines release, from sympathetic neurons and adrenal chromafin cells, which increase vasconstriction and reduces vasodilation, dramatically elevating peripheral vascular resistance, and arterial pressure. These findings may have important impact for antihypertensive pharmacotherapy due to multiple mechanisms involved in sympathetic hyperactivity associated to arterial hypertension, and potential risk involved in drug interaction between VACC blockers and drugs which increase [cAMP] c. New antihypertensive pharmacotherapy strategies could be developed in future to attenuate the cardiovascular risk associated with drug interference on the Ca2+/cAMP interaction in sympathetic neurons, and adrenal chromafin cells.
REFERENCES
- Grossman E, Messerli FH. Effect of calcium antagonists on sympathetic activity. Eur Heart J. 1998; 19: 27-31. Ref.: https://goo.gl/kv0mWq
- Kreye VA, Luth JB. Proceedings: Verapamil-induced phasic contractions of the isolated rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1975. Ref.: https://goo.gl/isGRSy
- French AM, Scott NC. A comparison of the effects of nifedipine and verapamil on rat vas deferens. Br J Pharmacol. 1981; 73: 321-323. Ref.: https://goo.gl/VPiQ7p
- Moritoki H, Iwamoto T, Kanaya J, Maeshiba Y, Ishida Y, et al. Verapamil enhances the non-adrenergic twitch response of rat vas deferens. Eur J Pharmacol. 1987; 140: 75-83. Ref.: https://goo.gl/AWCnbB
- Rae GA, Calixto JB. Interactions of calcium antagonists and the calcium channel agonist Bay K 8644 on neurotransmission of the mouse isolated vas deferens. Br J Pharmacol. 1989; 96: 333-340. Ref.: https://goo.gl/yo7i3y
- Bergantin LB, Souza CF, Ferreira RM, Smaili SS, Jurkiewicz NH, et al. Novel model for "calcium paradox" in sympathetic transmission of smooth muscles: role of cyclic AMP pathway. Cell Calcium. 2013; 54: 202-212. Ref.: https://goo.gl/wwbocE
- Bergantin LB, Jurkiewicz A, García AG, Caricati-Neto A. A Calcium Paradox in the Context of Neurotransmission. Journal of Pharmacy and Pharmacology. 2015; 3: 253-261. Ref.: https://goo.gl/zeaMXy
- Caricati-Neto A, García AG, Bergantin LB. Pharmacological implications of the Ca2+/cAMP signalling interaction: from risk for antihypertensive therapy to potential beneficial for neurological and psychiatric disorders. Pharmacol Res Perspect. 2015; 3: 181. Ref.: https://goo.gl/iEJcY9
- Pohar B, Grad A, Mozina M, Rakovec P, Horvat M. Paradoxical elevation of pulmonary vascular resistance after nifedipine in primary pulmonary hypertension. A case study. Cor et vasa. 1989; 31: 238-241. Ref.: https://goo.gl/IfAwve
- Ruzicka M, Coletta E, Floras J, Leenen FH. Effects of low-dose nifedipine GITS on sympathetic activity in young and older patients with hypertension. J Hypertens. 2004; 22: 1039-1044. Ref.: https://goo.gl/csJI90
- Elliott WJ, Ram CV. Calcium channel blockers. J Clin Hypertens.Greenwich. 2011; 13: 687-689. Ref.: https://goo.gl/xVvhxz
- Bergantin LB, Caricati-Neto A. Challenges for the pharmacological treatment of neurological and psychiatric disorders: Implications of the Ca2+/cAMP intracellular signalling interaction. Eur J Pharmacol. 2016; 788: 255-260. Ref.: https://goo.gl/FBjENG