More Information
Submitted: February 25, 2023 | Approved: March 15, 2023 | Published: March 16, 2023
How to cite this article: Fugasot CC, Rodríguez-Leor O, Sadurní JR, Fadeuilhe E, Martínez JP, et al. Renal denervation for resistant hypertension and heart failure with a reduced ejection fraction. Ann Clin Hypertens. 2023; 7: 001-003.
DOI: 10.29328/journal.ach.1001033
Copyright License: © 2023 Fugasot CC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Renal denervation for resistant hypertension and heart failure with a reduced ejection fraction
Carles Cañameras Fugasot1, Oriol Rodríguez-Leor2, Josep Riera Sadurní1, Edgar Fadeuilhe3, Javier Paul Martínez1, Marina Urrutia Jou1, Jordi Bover1 and Maribel Troya-Saborido4*
1Nephrology Department, Germans Trias I Pujol University Hospital (HGTiP) & REMAR-IGTP Group Institute Germans Trias I Pujol (IGTP), Can Ruti Campus, Badalona (Barcelona), Spain
2Heart Institute, Germans Trias i Pujol University Hospital (HGTiP), Badalona, Barcelona, Center for Biomedical Research in Cardiovascular Diseases Network (CIBERCV), Carlos III Health Institute, Madrid, Spain
3Heart Institute, Germans Trias i Pujol University Hospital (HGTiP), Badalona, Barcelona, Spain
4Nephrology Department, Germans Trias I Pujol University Hospital (HGTiP) & REMAR-IGTP Group Institute Germans Trias I Pujol (IGTP), Can Ruti Campus, Badalona (Barcelona), Medicine Department, Autonomous University of Barcelona (UAB), Barcelona, Spain
*Address for Correspondence: Maribel Troya-Saborido, Nephrology Department, Germans Trias I Pujol University Hospital (HGTiP) & REMAR-IGTP Group Institute Germans Trias I Pujol (IGTP), Can Ruti Campus, Badalona (Barcelona), Medicine Department, Autonomous University of Barcelona (UAB), Barcelona, Spain, Email: [email protected]
Hypertension is a risk factor for the development of heart failure and has a negative impact on the survival of these patients. Although patients with these two conditions usually take different antihypertensive medications, some patients do not achieve adequate blood pressure control and their hypertension becomes resistant or refractory. In this scenario, percutaneous renal denervation has emerged in recent years as an alternative to achieve blood pressure control goals. We present the case of a 53-year-old woman with a medical history of essential hypertension, hypercholesterolemia, unipolar depression, and diabetes, who was diagnosed with dilated cardiomyopathy with reduced left ventricular ejection fraction (33%). Despite the initiation of multiple antihypertensive medications and placement of a cardiac resynchronization therapy pacemaker, the patient remained hypertensive with a left ventricular ejection fraction of 40%. At that time, percutaneous renal denervation was performed without complications, and one year after the procedure, the patient had improved better blood pressure control and the left ventricular ejection fraction increased to 51%. This case illustrates one of the clinical scenarios in which it has been suggested that renal denervation may be more beneficial, as in the situation of patients with refractory hypertension and heart failure.
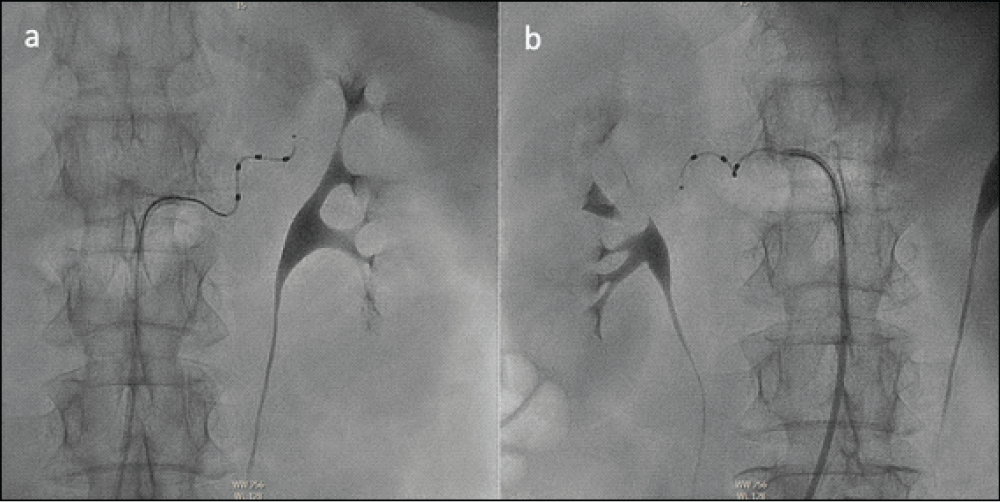
A 53-year-old woman with a medical history of essential hypertension, hypercholesterolemia, unipolar depression and type 2 diabetes mellitus was diagnosed with dilated cardiomyopathy due to chronic dyspnea. Renal function was normal [estimated glomerular filtration rate (CKD-EPI equation) of > 90 mL/min/1.73 m2], without albuminuria, and remained stable throughout the follow-up. The transthoracic echocardiography showed a severely dilated left ventricle (LV) with severe ventricular dysfunction [left ventricular ejection fraction (LVEF) 33% using Simpson’s biplane method] and the electrocardiogram showed sinus rhythm with left bundle branch block (QRS 150 ms). The cardiac magnetic resonance showed the same findings, with a severely dilated LV, non-hypertrophic, and LVEF of 29% with diffuse hypokinesia. No areas of necrosis, fibrosis or myocardial infiltration were found. Cardiac catheterization did not show coronary lesions. Over the next 3 years, multiple drugs were started, including beta-blockers, angiotensin receptor-neprilysin inhibitors, mineralocorticoid receptor agonists, calcium channel blockers, diuretics, ivabradine, digoxin, hydralazine, and dapagliflozin. Because of the persistence of reduced LVEF in spite of medical therapy, a cardiac resynchronization therapy pacemaker (CRT-P) was implanted. One year after the implantation of CRT-P, LVEF improved to 40%; however, despite full doses of all antihypertensive drugs the patient still had high blood pressure (BP), with an average of three standardized BP of 153/88 mmHg. In the presence of refractory hypertension, secondary causes were excluded and correct adherence to antihypertensive therapy and lifestyles was ensured. In addition, uncontrolled hypertension was confirmed with automated oscillometric blood pressure monitoring (AOBPM) (Table 1). Due to the patient’s very high cardiovascular risk, renal denervation (RDN) was proposed to achieve better BP control. The patient accepted the procedure, signed the informed consent took the risks of intervention, and was admitted electively. Under local anesthesia, through the right femoral artery, 8 radio frequency pulses were applied to the main trunk of the left renal artery and 7 radio frequency pulses to the main trunk of the right renal artery (Figure 1). More pulses in distal branches could not be applied due to anatomical difficulties. The procedure ended without complications and the patient was followed up in the hospital for one day. Immediately after the operation, her BP in the inpatient department decreased to 118/74 mmHg. One year after the RDN, a new AOBPM was performed and showed a reduction in BP, achieving a good BP according to guidelines (Table 1). Transthoracic echocardiography one year after RDN also showed improvement in LVEF, which was 51%.
Figure 1: Angiography of the Left (a) and Right (b) renal arteries during the process of renal denervation.
| Table 1: Data of automated oscillometric blood pressure monitoring before and one year after the RDN. | ||||
| Before RDN | After RDN | |||
| Time | Systolic BP [mmHg (SD)] |
Diastolic BP [mmHg (SD)] | Systolic BP [mmHg (SD)] | Diastolic BP [mmHg (SD)] |
| 11:00 | 147 | 86 | 138 | 86 |
| 12:00 | 145 | 84 | 148 | 103 |
| 13:00 | 118 | 70 | 134 | 92 |
| 14:00 | 93 | 55 | 125 | 77 |
| 15:00 | 135 | 72 | 131 | 77 |
| 16:00 | 167 | 71 | 130 | 79 |
| 17:00 | 133 | 60 | 129 | 71 |
| 18:00 | 141 | 77 | 134 | 77 |
| 19:00 | 149 | 82 | 134 | 104 |
| 20:00 | 143 | 69 | 131 | 71 |
| 21:00 | 123 | 60 | 131 | 81 |
| 22:00 | 125 | 65 | 139 | 78 |
| 23:00 | 147 | 81 | 140 | 73 |
| 0:00 | 140 | 66 | 117 | 60 |
| 1:00 | 127 | 60 | 123 | 62 |
| 2:00 | 135 | 62 | 125 | 67 |
| 3:00 | 154 | 74 | 128 | 69 |
| 4:00 | 139 | 66 | 129 | 70 |
| 5:00 | 127 | 69 | 133 | 64 |
| 6:00 | 135 | 68 | 128 | 67 |
| 7:00 | 128 | 77 | 152 | 73 |
| 8:00 | 154 | 80 | 155 | 88 |
| 9:00 | 155 | 81 | ||
| 10:00 | 167 | 88 | ||
| Overall | 140 (17.84) | 72 (10.11) | 133 (12.66) | 77 (12.27) |
| Wake period | 142 (19.90) | 74 (10.27) | 136 (13.59) | 82 (12.27) |
| Sleep period | 137 (12.55) | 68 (9) | 129 (9.86) | 68 (6.8) |
| RDN: Renal Denervation; BP: Blood Pressure; SD: Standard Deviation. | ||||
Hypertension is a risk factor for the development of heart failure (HF) with a negative impact on clinical evolution and also on the survival of these patients [1,2]. In addition, the treatment and control of hypertension have a significant impact on reducing hospitalization for HF [3]. For these reasons, excellent control of BP is considered essential for the prevention and control of HF [4]. Percutaneous RDN is an emergent invasive technique used to control BP that consists of generating heat injury to the nerves of the sympathetic nervous system located around the renal arteries using an endovascular catheter that generates radiofrequency waves [5]. Efferent renal nerves contribute to hypertension by causing renal vasoconstriction, vascular hypertrophy, stimulating renin release, and increasing renal sodium and water reabsorption. Afferent nerves cause reflex activation of the sympathetic axis directed to various tissues and vascular beds [6]. Initially, percutaneous RDN was considered a rescue treatment in patients with resistant hypertension to drug treatment [7]. However, in recent years, up to 4 randomized multicenter studies with a simulated control group (sham control) have robustly demonstrated the efficacy and safety of RDN in the treatment of patients with moderate hypertension with and without drug treatment. Although more studies are still needed to facilitate the selection of patients who could benefit from RDN, it has been proposed that patients with HF could be good candidates due to the improvement in the autonomic balance, the decrease in the activity of the renin-angiotensin system and the reduction of cardiac remodeling [8]. In summary, we present a patient with HF and refractory hypertension who presents a very high cardiovascular risk. After a successful RDN, BP was reduced and hypertension was well controlled.
- Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, Wainford RD, Williams B, Schutte AE. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020 Jun;75(6):1334-1357. doi: 10.1161/HYPERTENSIONAHA.120.15026. Epub 2020 May 6. PMID: 32370572.
- Kasiakogias A, Rosei EA, Camafort M, Ehret G, Faconti L, Ferreira JP, Brguljan J, Januszewicz A, Kahan T, Manolis A, Tsioufis K, Weber T, von Lueder TG, Smiseth OA, Wachtell K, Kjeldsen SE, Zannad F, Mancia G, Kreutz R. Hypertension and heart failure with preserved ejection fraction: position paper by the European Society of Hypertension. J Hypertens. 2021 Aug 1;39(8):1522-1545. doi: 10.1097/HJH.0000000000002910. PMID: 34102660.
- Beckett N, Peters R, Leonetti G, Duggan J, Fagard R, Thijs L, Narkiewicz K, McCormack T, Banya W, Fletcher A, Bulpitt C; HYVET Study Group. Subgroup and per-protocol analyses from the Hypertension in the Very Elderly Trial. J Hypertens. 2014 Jul;32(7):1478-87; discussion 1487. doi: 10.1097/HJH.0000000000000195. PMID: 24984177.
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members:. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018 Oct;36(10):1953-2041. doi: 10.1097/HJH.0000000000001940. Erratum in: J Hypertens. 2019 Jan;37(1):226. PMID: 30234752.
- Rodríguez-Leor O. Renal denervation for the management of hypertension. Joint position statement from the SHE-LELHA and the ACI-SEC. REC Interv Cardiol. 2022; 4:39-46.
- Schlaich MP, Schmieder RE, Bakris G, Blankestijn PJ, Böhm M, Campese VM, Francis DP, Grassi G, Hering D, Katholi R, Kjeldsen S, Krum H, Mahfoud F, Mancia G, Messerli FH, Narkiewicz K, Parati G, Rocha-Singh KJ, Ruilope LM, Rump LC, Sica DA, Sobotka PA, Tsioufis C, Vonend O, Weber MA, Williams B, Zeller T, Esler MD. International expert consensus statement: Percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013 Dec 3;62(22):2031-45. doi: 10.1016/j.jacc.2013.08.1616. Epub 2013 Sep 18. PMID: 24021387.
- Symplicity HTN-2 Investigators; Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010 Dec 4;376(9756):1903-9. doi: 10.1016/S0140-6736(10)62039-9. Epub 2010 Nov 17. PMID: 21093036.
- Barbato E, Azizi M, Schmieder RE, Lauder L, Böhm M, Brouwers S, Bruno RM, Dudek D, Kahan T, Kandzari DE, Lüscher TF, Parati G, Pathak A, Ribichini FL, Schlaich MP, Sharp ASP, Sudano I, Volpe M, Tsioufis C, Wijns W, Mahfoud F. Renal denervation in the management of hypertension in adults. A clinical consensus statement of the ESC Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2023 Feb 15:ehad054. doi: 10.1093/eurheartj/ehad054. Epub ahead of print. PMID: 36790101.