More Information
Submitted: September 05, 2024 | Approved: September 11, 2024 | Published: September 12, 2024
How to cite this article: Vyhmeister K, Gavaza P, Nguyen M, Kang G, Tran HN. Evaluation of Long-term Antithrombotic Management for Atrial Fibrillation Patients with a History of Coronary Stent Implantation. Ann Clin Hypertens. 2024; 8(1): 001-006. Available from: https://dx.doi.org/10.29328/journal.ach.1001035.
DOI: 10.29328/journal.ach.1001035
Copyright License: © 2024 Vyhmeister K, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Atrial fibrillation; Antithrombotic therapy; Percutaneous coronary intervention; Stents; Platelet aggregation inhibitors
RelationshipEvaluation of Long-term Antithrombotic Management for Atrial Fibrillation Patients with a History of Coronary Stent Implantation
Kirsten Vyhmeister1, Paul Gavaza2, Murphy Nguyen1, Grace Kang1 and Huyentran N Tran3*
1Loma Linda University School of Pharmacy, Loma Linda, CA, USA
2Department of Pharmaceutical and Administrative Sciences, Loma Linda University School of Pharmacy, Loma Linda, CA, USA
3Department of Pharmacy Practice, Loma Linda University School of Pharmacy, Loma Linda, CA, USA
*Address for Correspondence: Huyentran N Tran, PharmD, BCPS, BCCP, Department of Pharmacy Practice, Loma Linda University School of Pharmacy, Loma Linda, CA, USA, Email: [email protected]
Purpose: American expert consensus publications recommend discontinuation of antiplatelet agents 6 to 12 months after Percutaneous Coronary Intervention (PCI) in patients with Atrial Fibrillation (AF) who require chronic anticoagulation, and use of oral anticoagulant monotherapy thereafter. This study aimed to assess real-world long-term antithrombotic therapy management practices and factors associated with the continuation of antiplatelet agents past 12 months post-PCI in patients with AF requiring chronic anticoagulation.
Methods: Patients with AF and a history of PCI greater than 12 months before their most recent encounter with physicians at an outpatient electrophysiology clinic were identified by chart review. Patient demographics, clinical characteristics, and current antithrombotic regimen were collected from encounters that occurred between July 2019 and June 2022. The independent predictive factors associated with the continuation of antiplatelet agents were identified using univariate and regression analyses.
Results: Out of 66 patients, 67% continued antiplatelet therapy for greater than 12 months post-PCI. Patients on antiplatelets were significantly less likely to have bare metal stents (p = 0.006), be greater than five years post-PCI (p = 0.002), and have a HASBLED score of two or less (p = 0.028) when compared to patients on oral anticoagulant monotherapy. Bare metal stent history (p = 0.045) and HASBLED score of two or less (p = 0.016) were also significant in regression analysis.
Conclusion: This study found that most patients with AF and a history of PCI continued antiplatelet therapy longer than 12 months post-PCI, often despite the high bleeding risk.
Atrial Fibrillation (AF) is a condition that puts patients at high risk for stroke and systemic embolism, and chronic anticoagulation is often indicated to reduce that risk [1]. Approximately 20% to 40% of patients with AF also have a diagnosis of Coronary Artery Disease (CAD), which frequently leads to a need for Percutaneous Coronary Intervention (PCI) and coronary stent implantation [2]. Dual Antiplatelet Therapy (DAPT), consisting of aspirin and a P2Y12 inhibitor, followed by single antiplatelet therapy is the current recommendation for preventing stent thrombosis and ischemic events after PCI with stenting [3]. For patients who undergo PCI and have an indication for chronic anticoagulation, adding aspirin and a P2Y12 inhibitor to oral anticoagulation, referred to as triple therapy, is recommended initially, however, doing so significantly increases the risk of bleeding [1,4]. Several landmark trials exploring the optimal time frame and anticoagulant choice to use for triple therapy after PCI in patients on chronic anticoagulation [5-9]. Based on those trials, guidelines now recommend the use of direct oral anticoagulants over warfarin in most patients and recommend limiting triple therapy to the peri-PCI period or up to one-month post-PCI, and continuing double therapy with an oral anticoagulant and P2Y12 inhibitor thereafter [1,3,10].
After 6 to 12 months of antiplatelet therapy, European guidelines and American consensus publications recommend discontinuation of antiplatelet agents in patients with stable CAD who require chronic anticoagulation [10-13]. Those recommendations were initially based on expert opinion, but in the most recent guideline and consensus updates, new evidence is cited to support de-escalation to oral anticoagulant monotherapy after 12 months post-PCI [10,13,14]. The AFIRE trial was the first large, randomized trial to assess the long-term risks and benefits of oral anticoagulant monotherapy versus double therapy in patients with AF and stable CAD. Patients were randomized to either rivaroxaban monotherapy or rivaroxaban double therapy with either aspirin or a P2Y12 inhibitor and followed for a median of 24 months. Although the study did not limit its definition of stable CAD to patients who underwent PCI, approximately 65% of the patients in both the treatment and control groups had a history of PCI with stent placement greater than 1 year before enrollment. The primary composite efficacy endpoint of embolic events, ischemic events, or death from any cause showed that oral anticoagulant monotherapy was non-inferior to double therapy. Oral anticoagulant monotherapy was found to be superior to double therapy for the primary safety endpoint of major bleeding as defined by the International Society on Thrombosis and Hemostasis [15].
American guidelines have yet to provide recommendations for long-term antithrombotic therapy management in patients with AF who have undergone PCI with stenting, but in December 2020, the American College of Cardiology (ACC) published recommendations in their 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy that align with European guidelines and are supported by the AFIRE trial [1,3,14]. The ACC consensus publication recommends discontinuation of antiplatelet agents for patients on chronic anticoagulation who are greater than 12 months out from their PCI and who have not had a recurrent ischemic event post-PCI. Continuation of antiplatelet agents beyond 12 months is deemed reasonable if patients are at low bleed risk and have high ischemic risk [14].
Given the lack of American guideline recommendations on long-term antithrombotic therapy management and the allowance for antiplatelet continuation beyond 12 months post-PCI provided in American consensus publications and European guidelines, this study aimed to assess real-world long-term antithrombotic management practices in America at an outpatient electrophysiology clinic. The purpose of this study was to identify factors predicting a continuation of antiplatelet agents in the past 12 months post-PCI and to determine if the recently published ACC Expert Consensus Decision Pathway had any influence on the frequency of antiplatelet continuation past 12 months.
This retrospective, observational, case-control study assessed factors associated with the continuation of antiplatelet agents longer than 12 months post-PCI with coronary stenting in patients with AF who qualified for chronic anticoagulation. Case-control groups were determined based on the antithrombotic regimen documented at the most recent EP clinic encounter, with those on oral anticoagulant monotherapy serving as the controls, and those on any antiplatelet agent, regardless of whether they were taking an oral anticoagulant, referred to hereafter as ‘antiplatelet overtreated’, serving as cases. Case and control groups were further divided into patients seen before and after January 1, 2021, to assess the influence of recent American consensus statement publications on antithrombotic management practices. The study was conducted at Loma Linda University Health – International Heart Institute, and the study protocol was reviewed and approved by the Institutional Review Board under IRB # 5220233.
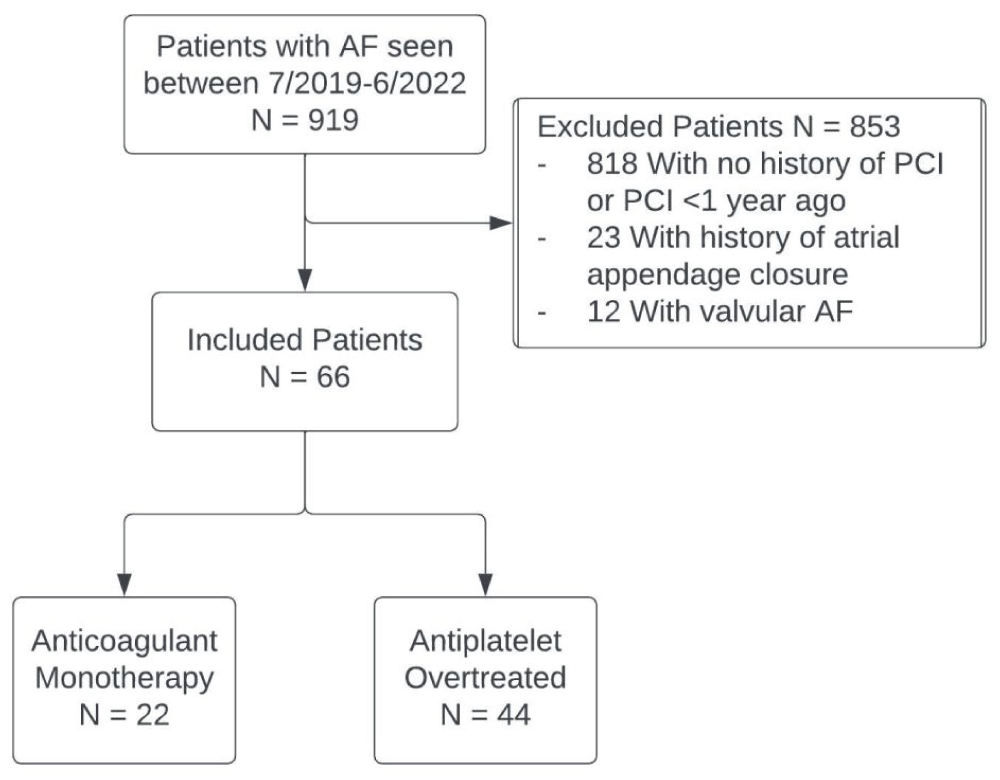
Patients 18 years and older with AF and a history of coronary stent implantation greater than 12 months before their most recent encounter with physicians at an outpatient electrophysiology clinic were identified by chart review (Figure 1). Patients with a history of valvular AF and atrial appendage closure were excluded. CHA2DS2-VASc score was used to confirm patients qualified for chronic anticoagulation. Patients were required to have a CHA2DS2-VASc score of 2 or greater for males and 3 or greater for females to be included in this study. Patient demographics (e.g., age, gender, body mass index), clinical characteristics, HASBLED score (assessing bleed risk in AF), and current antithrombotic regimen were collected from the patient’s most recent encounters that occurred between July 2019 and June 2022. The HAS-BLED score is used to assess the one-year risk of major bleeding in patients with AF who are on anticoagulation therapy. It includes parameters such as hypertension, abnormal renal or liver function, history of stroke, bleeding predisposition, labile INR, elderly age (≥ 65 years), and use of drugs or alcohol that may predispose to bleeding, with a score of 3 or more indicating high risk for bleeding [16].
Figure 1: Patient identification, exclusions, and grouping. AF indicates atrial fibrillation; PCI, and percutaneous coronary intervention.
Descriptive statistics were computed for all study variables. The independent predictive factors associated with the continuation of antiplatelet agents greater than 12 months post-PCI were identified using univariate and regression analyses. A two-sided alpha level of 0.05 was used for all statistical tests. Statistical analysis was performed using SPSS version 28.0 for Windows (IBM).
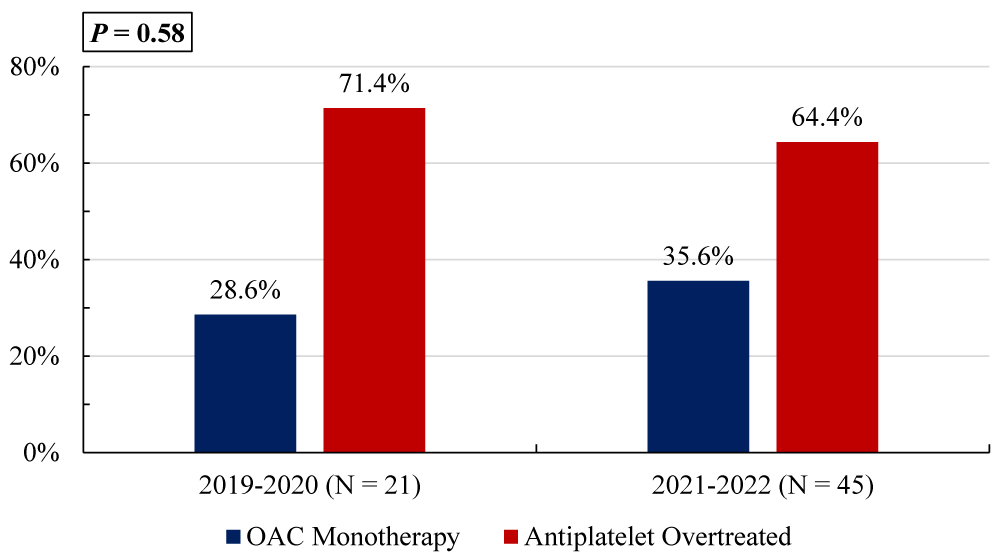
Out of 66 patients who met the study inclusion criteria, 67% (n = 44) continued antiplatelet therapy for greater than 12 months post-PCI. The majority of those who continued antiplatelets were receiving double therapy with an OAC and either aspirin or clopidogrel (Table 1). Four patients continued triple therapy with an OAC, aspirin, and clopidogrel, and eight patients were on dual or single antiplatelet therapy without any OAC. Most patients were taking direct oral anticoagulants such as apixaban and rivaroxaban, however, four patients were on warfarin monotherapy and two were on warfarin in addition to antiplatelet agents. There was no statistically significant difference in the percent of antiplatelet overtreated patients between those seen before and after January 1, 2021 (Figure 2).
Figure 1: Comparison of antithrombotic therapy management before and after January 1, 2021, in patients with atrial fibrillation and a history of percutaneous intervention (PCI) greater than 12 months prior.
| Table 1: Antithrombotic therapy by group. | |
| Antithrombotic Therapy | N (%) |
| Anticoagulant Monotherapy (n = 22) | |
| Apixaban | 16 (72.7) |
| Warfarin | 4 (18.2) |
| Rivaroxaban | 2 (9.1) |
| Antiplatelet Overtreated (n = 44) | |
| Apixaban + aspirin | 11 (25.0) |
| Apixaban + clopidogrel | 10 (22.7) |
| Rivaroxaban + aspirin | 7 (15.9) |
| Aspirin monotherapy | 4 (9.1) |
| Clopidogrel monotherapy | 3 (6.8) |
| Apixaban + aspirin + clopidogrel | 2 (4.5) |
| Apixaban + ticagrelor | 1 (2.3) |
| Dabigatran + aspirin | 1 (2.3) |
| Warfarin + clopidogrel | 1 (2.3) |
| Rivaroxaban + clopidogrel | 1 (2.3) |
| Aspirin + clopidogrel | 1 (2.3) |
| Rivaroxaban + aspirin + clopidogrel | 1 (2.3) |
| Warfarin + aspirin + clopidogrel | 1 (2.3) |
Patient demographics and past medical history were similar between the OAC monotherapy and antiplatelet overtreated groups with a few exceptions (Table 2). Antiplatelet overtreated patients were significantly less likely to have bare metal stents (p = 0.006), be greater than five years post-PCI (p = 0.002), and have a HASBLED score of two or less (p = 0.028) when compared to patients on OAC monotherapy. Bare metal stent history (p = 0.045) and HASBLED score two or less (p = 0.016) were also significant independent negative predictors of being antiplatelet overtreated, whereas greater than five years post-PCI was not (p = 0.065). These predictors accounted for 30.5% of the variance in the model.
| Table 2: Patient characteristics by group. | |||
| Characteristic | Anticoagulant Monotherapy (n = 22) |
Antiplatelet Overtreated (n = 44) |
p value |
| Age in years, mean ± SD | 76.1 ± 13.0 | 73.7 ± 9.0 | 0.390 |
| Male, No. (%) | 11 (50) | 32 (72.7) | 0.068 |
| BMI (kg/m2), mean ± SD | 30.7 ± 6.5 | 28.3 ± 5.0 | 0.100 |
| > 5 years post PCI, No. (%) | 17 (77.3) | 16 (36.4) | 0.002* |
| Bare metal stent, No. (%) | 9 (40.9) | 5 (11.4) | 0.006* |
| HASBLED score ≤ 2, No. (%) | 19 (86.3) | 26 (59.1) | 0.028* |
| Myocardial infarction, No. (%) | 11 (50.0) | 29 (65.9) | 0.212 |
| Stroke, No. (%) | 4 (18.2) | 16 (36.4) | 0.130 |
| Peripheral arterial disease, No. (%) | 4 (18.2) | 3 (6.8) | 0.210 |
| Venous thromboembolism, No. (%) | 4 (18.2) | 5 (11.4) | 0.467 |
| Hypertension, No. (%) | 22 (100) | 40 (90.9) | 0.293 |
| Heart failure, No. (%) | 14 (63.6) | 33 (75.0) | 0.336 |
| Diabetes mellitus, No. (%) | 8 (36.4) | 19 (43.2) | 0.595 |
| Major bleed, No. (%) | 3 (13.6) | 11 (25.0) | 0.354 |
| Abbreviations: BMI: Body Mass Index; PCI: Percutaneous Coronary Intervention Note: *p < 0.05 indicates statistical significance. |
|||
Nearly two-thirds of the patients in this study were on antiplatelet agents longer than 12 months post PCI, a third of whom had their PCI greater than five years ago before their most recent encounter. There was a slight shift toward the use of oral anticoagulant monotherapy in the group with encounters after the ACC Expert Consensus Decision Pathway publication, but the change was not statistically significant. The finding that some patients were on triple therapy indicates that either guidelines and consensus publications are not being consistently applied to antithrombotic management or that there is a hesitancy to de-prescribe antiplatelet agents, which were often initiated by providers that were not associated with the EP clinic. There are several potential reasons for the continuation of antiplatelet therapy beyond 12 months post-PCI in patients with AF requiring chronic anticoagulation. One possible explanation is that physicians may not consistently adhere to established guidelines, either due to a lack of familiarity with the most up-to-date recommendations or a preference for long-standing practices. Additionally, the management of these patients often involves complex cases with multiple comorbidities or heightened thrombotic risk, leading to more cautious, extended treatment strategies. Another important factor could be the need for better collaboration and communication between electrophysiology and interventional cardiology teams, as differing treatment approaches may result in prolonged therapy without a clear plan for de-escalation. However, there is a notable lack of data on the rationale behind physicians’ decisions to continue antiplatelet therapy beyond the recommended duration, making it difficult to fully understand the drivers of overtreatment in this population. This highlights the need for further research into physician decision-making and adherence to guidelines in this context.
The imbalance in sample sizes between the two periods—21 patients before January 1, 2021, and 45 patients after—may impact the interpretation of the results regarding the antiplatelet overtreatment rates. Smaller sample sizes generally result in less statistical power, increasing the risk of a type II error, where a true difference may exist but is not detected as statistically significant. In this case, the smaller group (n = 21) could lead to an underestimation of differences between the two time periods, as variability in the data is less reliably captured with fewer patients. Additionally, the larger post-2021 group (n = 45) may provide a more accurate reflection of overtreatment trends, but the overall conclusion may still be skewed by the imbalance. As such, while we found no statistically significant difference in overtreatment rates, this result should be interpreted with caution. Further studies with more balanced groups or larger sample sizes would be necessary to confirm these findings.
Antiplatelet overtreated patients were significantly more likely to have HASBLED scores greater than two, which is expected given that antiplatelet use adds one point to a patient’s score. Many of the criteria in the HASBLED score such as stroke and age greater than 65 overlap with CHA2DS2-VASc score criteria, so patients with high calculated bleed risk may have high calculated thromboembolic risk as well. Generally, a HASBLED score of greater than two indicates a high risk for major bleeding, and alternatives to anticoagulation should be considered for AF patients. A high HASBLED score is not an absolute contraindication to anticoagulation though, and many of the criteria are modifiable risk factors [1]. Although the HASBLED score is indicated to help make decisions about anticoagulation in AF, it is reasonable to assume a score of greater than two on the HASBLED score would also make patients poor candidates for long-term use of antiplatelet agents. Based on this assumption, 41% of the patients in the antiplatelet overtreated group may have benefited from antiplatelet discontinuation at 12 months post-PCI to reduce the risk of major bleeds [17,18].
Lack of bare metal stents was also found to be an independent predictor of antiplatelet continuation beyond 12 months post-PCI. Bare metal stents are at lower risk for stent thrombosis and guidelines recommend a shorter duration of DAPT than for drug-eluting stents [3]. In the 2010 European AF guidelines, recommendations for the duration of antiplatelet therapy after PCI were stratified by HASBLED score, the setting of the procedure (elective versus acute coronary syndrome), and stent type, with bare metal stent implantations requiring the shortest durations of triple and double therapy [19]. Providers may feel more confident de-escalating antiplatelet therapy to double therapy and then anticoagulant monotherapy after bare metal stents due to the lower stent thrombosis risk. Bare metal stents are not commonly placed anymore, so patients with bare metal stents often had their stents placed 10 years or more before their most recent encounter. Patients with more remote stent history may be perceived as having lower ischemic risk and there are more opportunities for antiplatelet discontinuation over time, whether intentional or accidental.
The findings from this study have several important clinical implications, particularly for cardiologists and electrophysiologists. The fact that nearly two-thirds of patients remained on antiplatelet agents beyond 12 months post-PCI, including a significant proportion more than five years after PCI, suggests potential overtreatment with antithrombotic therapy. This prolonged therapy increases the risk of bleeding without clear evidence of additional benefit, especially for those on triple therapy [20-27]. The results highlight a need for greater adherence to guidelines and more routine reassessments of antithrombotic therapy, ensuring that patients are not unnecessarily exposed to risks. Additionally, the slight but non-significant shift toward oral anticoagulant monotherapy following the ACC Expert Consensus Decision Pathway publication suggests that guideline updates alone may not be sufficient to drive practice changes. Enhanced collaboration between interventional cardiologists, electrophysiologists, and other providers who initiate and manage these therapies is essential to optimize patient care and reduce unnecessary bleeding risks. Incorporating more robust systems for reassessment and de-prescribing may be crucial to aligning clinical practice with current evidence and recommendations.
This study was limited due to being conducted at a single center and does not necessarily represent general trends in antithrombotic therapy management in America. Due to the small sample size, regression analysis was limited to factors found to be significant in univariate analysis and no sub-analyses could be run. Another limitation is that medical history and medication reconciliations were constrained to what could be found in the patient’s electronic health record and may not have been complete. Finally, causality cannot be inferred from this study because of its retrospective and observational design.
The majority of patients evaluated in this study were continued on antiplatelet agents in addition to oral anticoagulation past 12 months post PCI, often despite high bleed risk. Bare metal stent implantation and HASBLED score of two or fewer were independent predictors of antiplatelet discontinuation and use of oral anticoagulation monotherapy, which aligns with European guidelines and consensus recommendations. This study found that American consensus recommendations, European guidelines, and the AFIRE trial have not shifted long-term antithrombotic management practice towards favoring oral anticoagulant monotherapy after 12 months post-PCI in patients with AF requiring chronic anticoagulation. Future randomized controlled trials in American populations should be conducted and American guideline recommendations should be developed to provide more certain guidance to providers on the long-term management of antithrombotic therapy in this population.
Key points
- A retrospective study was conducted at an electrophysiology clinic to assess the long-term management of antithrombotic therapy for patients with atrial fibrillation and a history of Percutaneous Intervention (PCI) with coronary stenting.
- The majority of patients continued antiplatelet agents greater than 12 months post-PCI.
- History of a bare metal stent and a HASBLED score of 2 or less were found to independently predict discontinuation of antiplatelets and use of anticoagulant monotherapy for patients greater than 12 months post-PCI.
- January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons [published correction appears in Circulation. 2019;140(6)]. Circulation. 2019;140(2). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000665
- Capodanno D, Huber K, Mehran R, Lip GYH, Faxon DP, Granger CB, et al. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(1):83-99. Available from: https://www.jacc.org/doi/10.1016/j.jacc.2019.05.016
- Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published correction appears in Circulation. 2022;145(11). Circulation. 2022;145(3). Available from: https://www.ahajournals.org/doi/10.1161/CIR.0000000000001038
- van Rein N, Heide-Jørgensen U, Lijfering WM, Dekkers OM, Sørensen HT, Cannegieter SC. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139(6):775-786. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.036248
- Dewilde WJM, Oirbans T, Verheugt FW, Kelder JC, De Smet BJGL, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381(9872):1107-1115. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)62177-1/fulltext
- Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423-2434. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1611594
- Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513-1524. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1708454
- Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394(10206):1335-1343. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31872-0/fulltext
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509-1524. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1817083
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC [published correction appears in Eur Heart J. 2021;42(5):507. Eur Heart J. 2021;42(5):373-498. Available from: https://doi.org/10.1093/eurheartj/ehaa612
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. Available from: https://doi.org/10.1093/eurheartj/ehw210
- Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective-2018 update. Circulation. 2018;138(5):527-536. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.118.034722
- Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, et al. Antithrombotic therapy in patients with atrial fibrillation treated With oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143(6):583-596. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.050438
- Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(5):629-658. Available from: https://www.jacc.org/doi/10.1016/j.jacc.2020.09.011
- Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381(12):1103-1113. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1904143
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-100. Available from: https://journal.chestnet.org/article/S0012-3692(10)60352-7/fulltext
- Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103-1113. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1904143
- Matsumura-Nakano Y, Shizuta S, Komasa A, Morimoto T, Masuda H, Shiomi H, et al. Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139:604-616. Available from: https://doi.org/10.1161/CIRCULATIONAHA.118.036768
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31(19):2369-2429. Available from: https://doi.org/10.1093/eurheartj/ehq278
- Dewilde WJM, Oirbans T, Verheugt FWA, Kelder JC, De Smet BJGL, Herrman J-P, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107-1115. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)62177-1/fulltext
- Fiedler KA, Maeng M, Mehilli J, Schulz-Schupke S, Byrne RA, Sibbing D, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol. 2015;65:1619-1629. Available from: https://www.jacc.org/doi/10.1016/j.jacc.2015.01.053
- Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423-2434. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1611594
- Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513-1524. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1708454
- Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509-1524. Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa1817083
- Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335-1343. Available from: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31872-0/fulltext
- Hoshi T, Sato A, Hiraya D, Watabe H, Takeyasu N, Nogami A, et al. Short-duration triple antithrombotic therapy for atrial fibrillation patients who require coronary stenting: results of the SAFE-A study. EuroIntervention. 2020;16–e172. Available from: https://eurointervention.pcronline.com/article/short-duration-triple-antithrombotic-therapy-for-atrial-fibrillation-patients-who-require-coronary-stenting-results-of-the-safe-a-study
- Smits PC, Frigoli E, Tijssen J, Juni P, Vranckx P, Ozaki Y, et al. Abbreviated antiplatelet therapy in patients at high bleeding risk with or without oral anticoagulant therapy after coronary stenting: an open-label, randomized, controlled trial. Circulation. 2021;144:1196-1211. Available from: https://doi.org/10.1161/CIRCULATIONAHA.121.056680